Description
This medicine may not be right for you. Read the label before purchase. Follow the directions for use. Incorrect use could be harmful. If symptoms persist, talk to your healthcare professional.
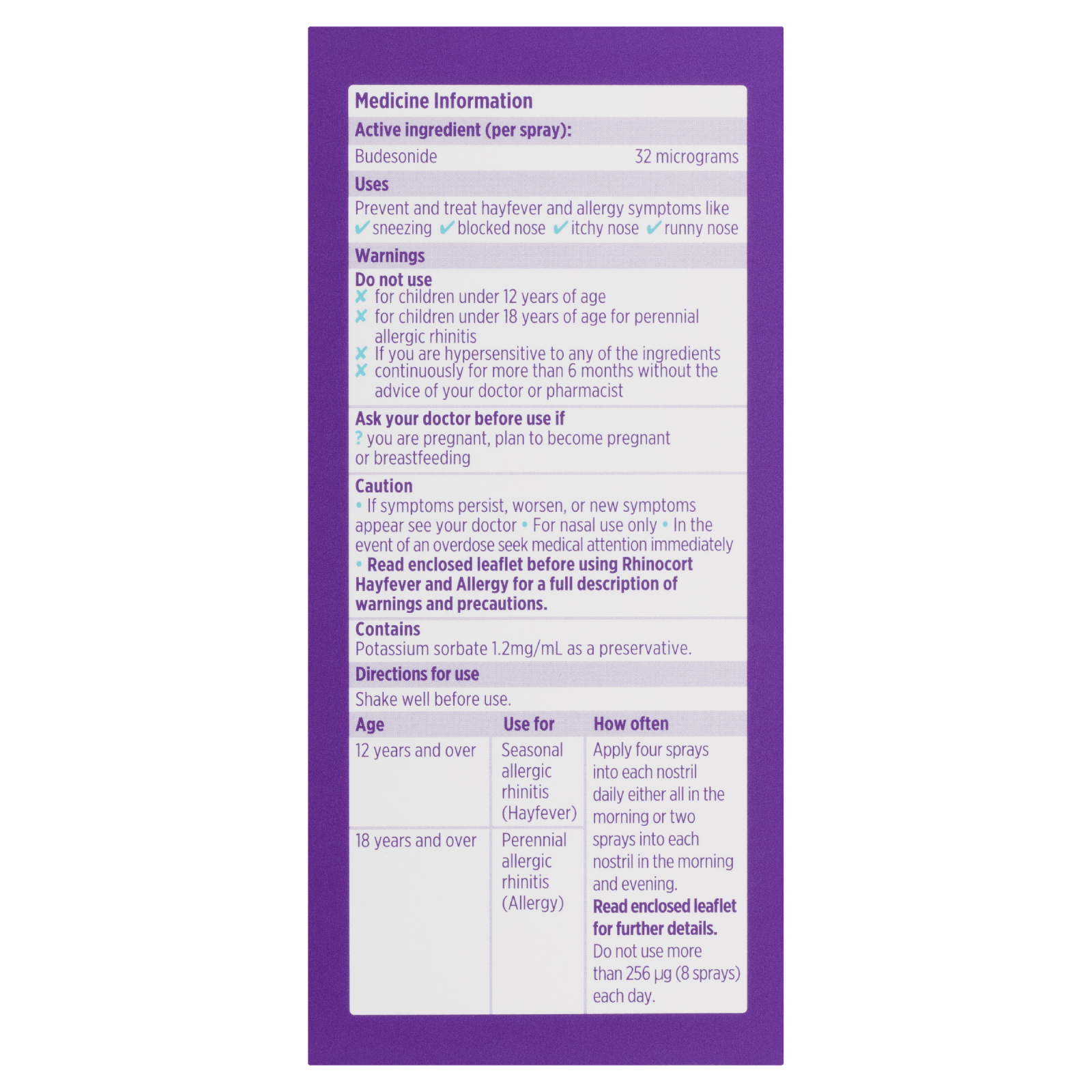
Product Information
Rhinocort Original Hayfever & Allergy Non-Drowsy Nasal Spray helps to prevent and relieve sneezing, blocked, runny or itchy nose caused by hayfever and other allergies. Comes in a 120 spray bottle. Apply 4 sprays in each nostril one per day.
Directions: Shake well before use.
Age: 12 years and over use for: Seasonal allergic rhinitis (Hayfever).
Age: 18 years and over use for: Perennial allergic rhinitis (Allergy)
How often: Apply four sprays into each nostril daily either all in the morning or two sprays into each nostril in the morning and evening. Read enclosed leaflet for further details. Do not use more than 256μg (8 sprays) each day.
Usage Advice: For best results, Rhinocort should be used regularly to keep hayfever and allergy symptoms under control. Using Rhinocort 1-2 days prior to exposure to pollen and allergens can help prevent symptoms from occurring. Use only if carton seal is unbroken. Storage Instructions: Store below 30°C. Do not freeze.
Warnings
Product Warnings:
Do not use:
– for children under 12 years of age.
– for children under 18 years of age for perennial allergic rhinitis.
– if you are hypersensitive to any of the ingredients.
– continuously for more than 6 months without the advice of your doctor.
Ask your doctor before use if:
– you are pregnant, plan to become pregnant or breastfeeding.
Ingredients
Active Ingredients:
– Budesonide (32ug per spray) is a corticosteroid that helps to prevent and treat hayfever and allergy symptoms by reducing swelling in the nasal passages.












Reviews
There are no reviews yet.